Details of the Drug
General Information of Drug (ID: DMY0UEQ)
| Drug Name |
VATALANIB
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vatalanib; 212141-54-3; Vatalanib base; N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin-1-amine; PTK787; Pynasunate; CGP 79787; Vatalanib free base; PTK-787; Vatalanib (free base); Vatalinib; ZK-232934; CGP-79787; ZK222584; PTK/ZK; CHEMBL101253; N-(4-Chlorophenyl)-4-(4-pyridinylmethyl)-1-phthalazinamine; UNII-5DX9U76296; CHEBI:90620; YCOYDOIWSSHVCK-UHFFFAOYSA-N; 1-(4-chloroanilino)-4-(4-pyridylmethyl)phthalazine; 5DX9U76296; NCGC00181350-01; 1-Phthalazinamine,N-(4-chlorophenyl)-4-(4-pyridinylmethyl)-; DSSTox_CID_26919
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
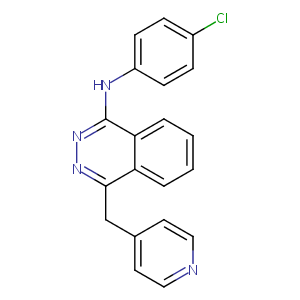 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 346.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
